News
The Greek Patients’ Association Launches Greece’s First EUPATI Patient Academy
The Greek Patients’ Association launches the first EUPATI Patient Academy in Greece (https://academy.greekpatient.gr/), a comprehensive educational program aimed at strengthening the role of patients in pharmaceutical research, clinical trials, and health policy development. Empowering patients through continuous education and access to reliable information can play a decisive role in shaping a modern, equitable, and efficient healthcare system—one in which patients’ voices have a meaningful impact not only on decisions regarding their treatment and care, but also on the improvement of services and the design of effective health policies. In this context, HACRO supports initiatives that promote the active participation ...
Read More >
Three dynamic tools by Rare Diseases Greece: Empowering patients with Rare Diseases
Rare Diseases Greece presents three groundbreaking, interactive tools that offer valuable support to individuals living with rare diseases in Greece. These tools aim to improve access to therapies, advocate for patient rights, and enhance understanding of the rare disease landscape in the country. These tools promote transparency and empower citizens to actively participate in the management of their health and care. More information is available on the official website of Rare Diseases Greece: rarediseasesgreece.gr
Read More >
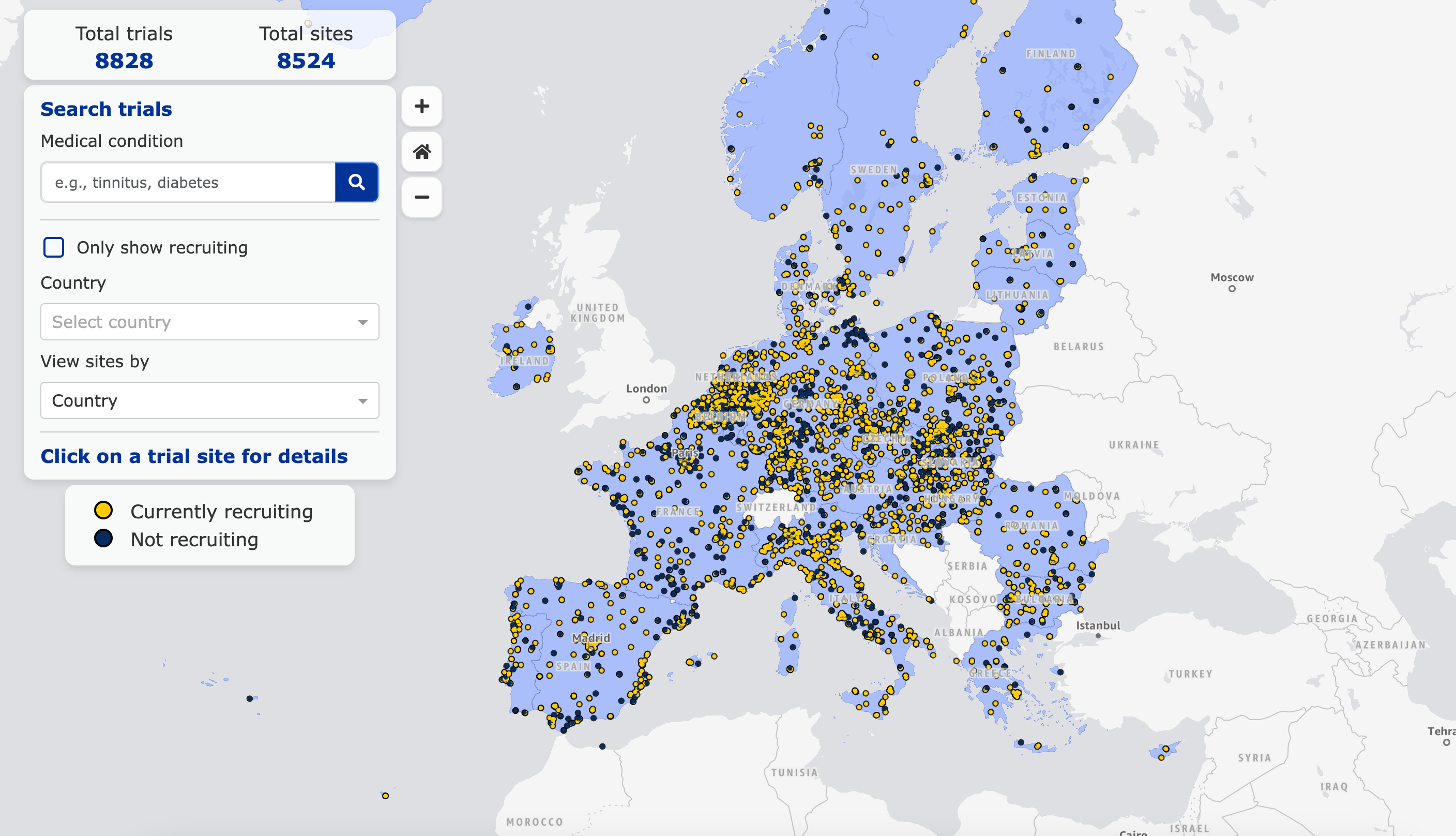
EMA’s New Clinical Trial Map: Transforming Access and Transparency in European Clinical Research
The new Clinical Trial Map, made available by the European Medicines Agency (EMA) through the public online platform CTIS, represents a particularly significant advancement in the field of clinical research in Europe. This innovative and effective tool enables patients and healthcare professionals to identify, in real-time, ongoing clinical trials across Europe by geographic region and medical condition, along with relevant investigator details, thus providing comprehensive information and facilitating enrollment opportunities for interested parties. The Clinical Trial Map reflects Europe’s goal of advancing clinical research across the continent and underscores its commitment to transparency in clinical trials. https://euclinicaltrials.eu/search-for-clinical-trials/trial-map/?lang=en
Read More >