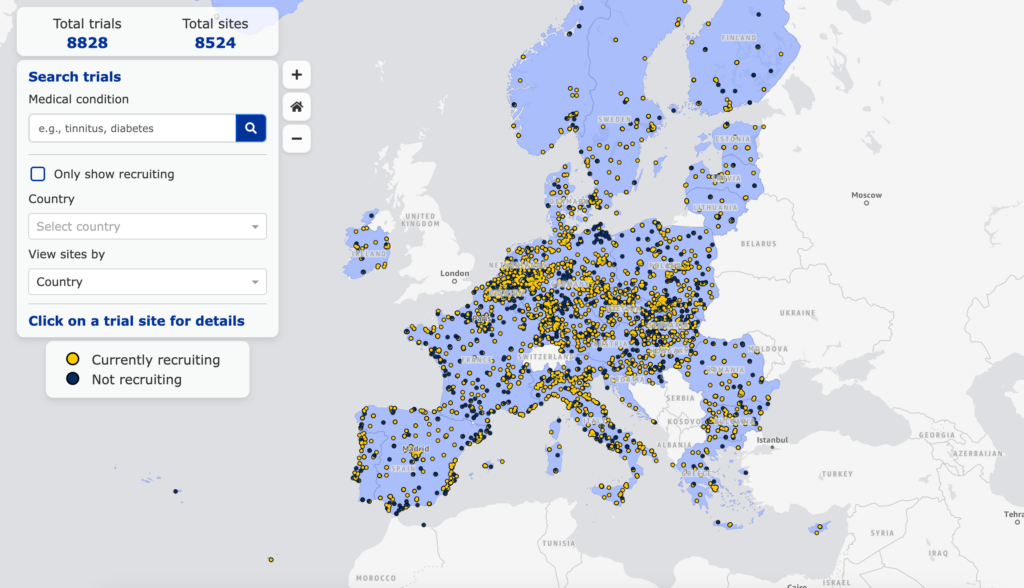
The new Clinical Trial Map, made available by the European Medicines Agency (EMA) through the public online platform CTIS, represents a particularly significant advancement in the field of clinical research in Europe.
This innovative and effective tool enables patients and healthcare professionals to identify, in real-time, ongoing clinical trials across Europe by geographic region and medical condition, along with relevant investigator details, thus providing comprehensive information and facilitating enrollment opportunities for interested parties.
The Clinical Trial Map reflects Europe’s goal of advancing clinical research across the continent and underscores its commitment to transparency in clinical trials.
https://euclinicaltrials.eu/search-for-clinical-trials/trial-map/?lang=en